論文がアクセプトされました(堀川先生)
Correlation between Soft X-ray Absorption and Emission Spectra of the Nitrogen
Atoms within Imidazolium-Based Ionic Liquids
Y. Horikawa, T. Tokushima, Osamu Takahashi, Hiroshi Hoke, T. Takamuku
J. Phys. Chem. B, 2016, 120, 7480–7487.
DOI : 10.1021/acs.jpcb.6b04132
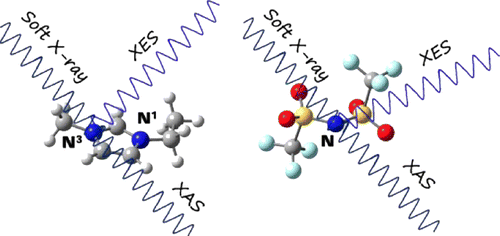
Abstract: Soft X-ray absorption spectroscopy (XAS) has been performed on the N K-edge
of two imidazolium-based ionic liquids (ILs), 1-ethyl-3-methylimidazolium
bis(trifluoromethylsulfonyl)amide ([C2mim][TFSA]) and 1-ethyl-3-methylimidazolium
bromide ([C2mim][Br]), to clarify the electronic structures of the ILs.
Soft X-ray emission spectroscopy (XES) has also been applied to the ILs
by excitation at various X-ray energies according to the XAS spectra. It
was possible to fully associate the XAS peaks with the XES peaks. Additionally,
both XAS and XES spectra of the ILs were well reproduced by the theoretical
spectra for a single-molecule model on [C2mim]+ and [TFSA]− using density
functional theory. The assignments for the XAS and XES peaks of the ILs
were accomplished from both experimental and theoretical approaches. The
theoretical XAS and XES spectra of [C2mim]+ and [TFSA]− did not significantly
depend on the conformations of the ions. The reproducibility of the theoretical
spectra for the single-molecule model suggested that the interactions between
the cations and anions are very weak in the ILs, thus scarcely influencing
the electronic structures of the nitrogen atoms.