論文がアクセプトされました(村藤先生・石黒先生・上條先生 共著)
Synthesis and Structural Characterization of Diazulenylborinic Acid
T. Murafuji, K. Shintaku, K. Nagao, Y. Mikata, K. Ishiguro, S. Kamijo
Heterocycles, 2017, 94, 2676-690.
DOI: 10.3987/COM-17-13651
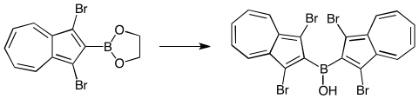
Abstruct: Diazulenylborinic acid 1 was synthesized by the reaction of 1,3-dibromoazulen-2-yllithium
(2) with 1,3-dibromoazulen-2-ylboronic acid ethylene glycol ester (7d).
The X-ray crystallographic study of 1 revealed that it forms a dimeric
structure through intermolecular hydrogen bonding between the hydroxyl
groups. To obtain the tetracoordinate borinate derivative from 1, the attempted
esterification of 1 with N,N-dimethylethanolamine did not give corresponding
borinate 8 but resulted in the unexpected protodeboronation to give parent
1,3-dibromoazulene (4). The 13C and 11B NMR studies of 1 in the presence
of Et3N revealed the change in the π-polarization of the azulenyl group
accompanied by the change in the geometry of the boron to a tetracoordinate
structure.