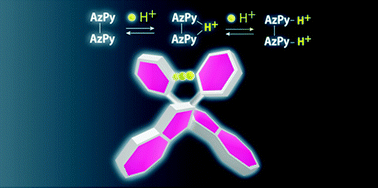
論文がアクセプトされ表紙を飾りました(綱島先生・村藤先生 共著)

Synthesis and Acid–Base Properties of a Proton-Bridged Biaryl CompoundBased
on Pyridylazulene
New J. Chem., 2015, 39, 9079-9085
K. Ninomiya, Y. Harada, T. Kanetou, Y. Suenaga, T. Murafuji, R. Tsunashima
DOI : 10.1039/c5nj01651g
Abstract: Herein, we report the synthesis of 1,1′-bi(2-pyridylazulene) (1), in which
pyridyl moieties were coupled to a biaryl framework for hydrogen bonding
between the two aryl skeletons. The two 2-pyridylazulene moieties in 1
were linked through facile aryl–aryl coupling between the 1- and 1′-positions
of the azulene skeletons, where 1-haloazulene was stabilized by electron
withdrawing pyridyl substitution. In addition, single crystals of the mono-protonated
species (1H+) were successfully obtained as the BF4− salt. X-ray diffraction
analysis at 153 K revealed an intramolecular hydrogen bond between the
two pyridyl moieties, giving a racemic mixture of axial chiral species.
DFT calculations were performed to understand the hydrogen bonding structure
and an almost single minimum potential for thermal proton motion was suggested.
The acid–base characteristics were investigated in acetonitrile and 1 was
revealed to exhibit two-step protonation of its pyridyl moieties. By comparison
with monomeric 2-pyridylazulene, the stronger basic character of 1 was
confirmed. This is ascribed to the macrocyclic effect of the two pyridyl
moieties bridged by a proton, as seen in the single crystal of 1H+. Furthermore,
two different energy shifts associated with intramolecular transition were
observed under protonation; the first protonation produced a blue shift
in the absorption maximum, whereas a red shift was observed for the second
protonation. The unusual blue shift was explained by the stabilization
of the HOMO owing to an extended electronic structure between the two azulene
skeletons. This unique steric structure was achieved by proton bridging
in the pyridyl-substituted biaryl compound.