論文がアクセプトされました(上條先生・石黒先生・村藤先生 共著)
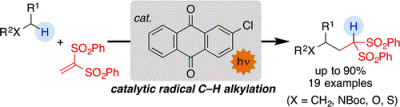
Alkylation of Nonacidic C(sp3)–H Bonds by Photoinduced Catalytic Michael-Type
Radical Addition
S. Kamijo, G. Takao, K. Kamijo, T. Tsuno, K. Ishiguro, T. Murafuji
Org. Lett., 2016, 18, 4912-4915
DOI: 10.1021/acs.orglett.6b02391
Abstruct: Photoinduced catalytic Michael-type radical addition was achieved via
olefin insertion into a nonacidic C(sp3)–H bond, utilizing 2-chloroanthraquinone
as a C–H bond-cleaving catalyst and 1,1-bis(phenylsulfonyl)ethylene as
an olefinic substrate. The present radical protocol allows carbon chain
extension stemming from nonacidic C–H bonds, which complements alkylation
at acidic C–H bonds under ionic conditions and installs the active methine
site that acts as a versatile synthetic handle for further transformations.