論文がアクセプトされました(綱島先生)
Solid-state Structure and Electronic States of Hydrogen Bonded-dimer of
Pyridyl-substituted Tetrathiafulvalene Salted with PF6−
T. Kanetou, R. Tsunashima, N. Hoshino, T. Akutagawa
RSC Adv., 2017, 7, 6236-6241.
DOI : 10.1039/C6RA27814K
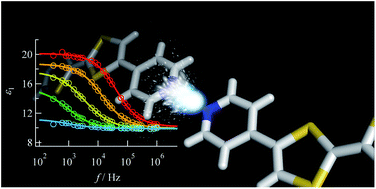
Abstract: Hydrogen bonding was investigated in a newly prepared salt of pyridyl-substituted
tetrathiafulvalene (TTFPy) that formed a dimer structure by hydrogen bonding
and was salted with PF6− anions, namely, (TTFPy2H)PF6 (1). Structural analysis
of the salt by single-crystal X-ray diffraction and temperature-dependent
FTIR spectra and measurements of its electrical conductivity and dielectric
properties were performed. Thermal fluctuations were investigated for protons
between two TTFPy molecules. An anisotropic dielectric anomaly was observed
owing to local proton transfer between two pyridyl moieties. This behavior
was well explained by a model of Debye-type dipole relaxation. In addition,
DFT calculations showed that the dipole originated from local proton transfer,
but the dipole moment in a network of TTFPy⋯H+⋯TTFPy dimers was amplified
by a factor of 2 owing to coupling with the electronic polarization of
TTFPy moieties.