論文がアクセプトされました(川俣先生・鈴木先生 共著)
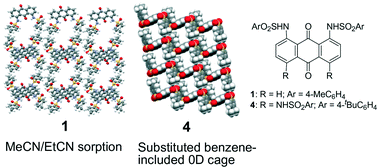
Selective MeCN/EtCN Sorption and Preferential Inclusion of Substituted
Benzenes in a Cage Structure with Arylsulfonamide-Armed Anthraquinones
T. Takeda, S. Noro, T. Nakamura, Y. Suzuki, J. Kawamata, T. Akutagawa
CrystEngComm, 2018, 20, 17-24 .
DOI: 10.1039/c7ce01752a
Abstruct: We report a series of crystal structures of arylsulfonamide-armed anthraquinones
(AQs) (1–4). The arylsulfonamide-armed AQs formed orthogonal aromatic arrangements
between the AQ unit and terminal aryl units due to well-defined intramolecular
hydrogen bonding between the carbonyl units of AQs and the amino groups
of sulfonamide units. Three disubstituted AQs 1–3 formed fundamental dimer
structures, which were stabilized by intermolecular π–π interaction between
AQs. Subtle differences in the dimer structures led to different packing
structures. Among them, the 1,8-bis(arylsulfonamide) derivative (1) formed
solvated crystals of 1·(MeCN), which exhibited reversible and selective
MeCN and/or EtCN adsorption–desorption behavior. Tetra(arylsulfonamide)
AQ (4) with four bulky substituents on its periphery formed various host–guest
molecular crystals of 4·X2 (X = toluene, xylene, trimethylbenzenes, 1,2,3,5-tetramethylbenzene,
anisole, and benzonitrile) with a rectangular zero-dimensional cage surrounded
by the π-planes of 4.