論文がアクセプトされました(川俣先生・鈴木先生 共著)
The Emergent Intramolecular Hydrogen Bonding Effect on the Electronic Structures
of Organic Electron Acceptors
T. Takeda, Y. Suzuki, J. Kawamata, S. Noro, T. Nakamura, T. Akutagawa
Phys. Chem. Chem. Phys., 2017, 19, 23905-23909.
DOI: 10.1039/C7CP04402J
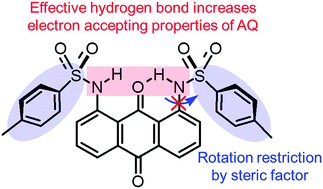
Abstruct: A new strategy for controlling the electron-accepting ability of an anthraquinone
(AQ)-based π-molecular system is proposed to take advantage of intramolecular
hydrogen bonding interactions. The electron-accepting properties of AQ
are enhanced by the introduction of bulky arylsulfonamide groups into AQ
derivatives due to the formation of effective intramolecular N–H⋯O hydrogen
bonding interaction and stabilization of the anion radical state even in
solution.