論文がアクセプトされました(堀川先生)
Effect of Amino Group Protonation on the Carboxyl Group in Aqueous Glycine
Observed By O1s X-Ray Emission Spectroscopy
Y. Horikawa, T. Tokushima, O. Takahashi, Y. Harada, A. Hiraya, S. Shin
Phys. Chem. Chem. Phys., 2018, 20, 23214-23221.
DOI : 10.1039/C7CP08305J
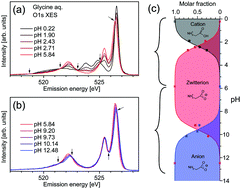
Abstract: The valence electronic structures of the amino acid glycine in aqueous
solution were investigated in detail through X-ray emission spectroscopy
at O 1s excitation under selective excitation conditions of the C[double
bond, length as m-dash]O site in the carboxyl group. The X-ray emission
spectra of glycine were similar to that of acetic acid (CH3COOH), suggesting
a resemblance between the molecular orbitals associated with the carboxyl
groups in the two molecules. The changes of O 1s X-ray emission spectra
as a function of pH were investigated in detail. In addition to spectral
changes due to protonation/deprotonation of the carboxyl group for lower
pH-values around the pKa value (∼2.3), the spectra of glycine exhibited
further changes in the higher-pH region near the pKb value of glycine (dissociation
constant of amino group ∼9.5). These results show the effects of amino
group protonation on the electronic state around the carboxyl group. X-ray
emission spectroscopy might be a tool to investigate intramolecular interactions
between functional groups in a molecule.