論文がアクセプトされました(山崎先生)
Factors Affecting Photocatalytic Activity of Visible Light-Responsive Titanium
Dioxide Doped with Chromium Ions
N. Nishiyama, K. Kozasa, T. Okajima, M. Fujitsuka, T. Majima, S. Yamazaki
Catal. Sci. Technol., 2018, 8, 4726-4733.
DOI: 10.1039/c8cy01411f
Abstract: Titanium dioxide doped with Cr ions (Cr-TiO2) was synthesized by a sol-gel
method, with only water as the solvent, and dialysis. For Cr-TiO2 sintered
at 200 °C, ca. 90% of 4-chlorophenol (4-CP) was degraded at Cr doping amounts
of 0.68–1.3 atom% under visible light irradiation. At doping amounts above
1.7 atom%, the 4-CP degradation ratio increased for Cr–TiO2 sintered at
300–500 °C. X-ray photoelectron spectroscopy and X-ray absorption near
edge structure measurements showed that only Cr(III) was present on the
surfaces of Cr–TiO2 samples sintered at 200 or 400 °C and some of the Cr(III)
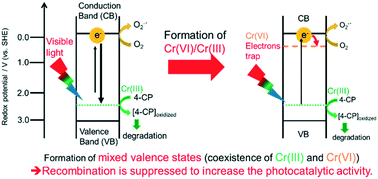
in the bulk was oxidized to Cr(VI) by sintering at 400 °C. The 4-CP degradation
was enhanced with increasing Cr(VI)/Cr(III) ratio in the bulk, suggesting
the spatial separation of Cr(III) on the surface under visible light irradiation:
electrons are excited from Cr(III) to the conduction band of TiO2 and then
trapped at the doped Cr(VI) whereas the photogenerated Cr(IV) oxidizes
4-CP. Time-resolved diffuse reflectance spectroscopy was used to evaluate
the lifetimes of the photogenerated electrons. The highest photocatalytic
activity was obtained with ca. 0.8 atom% Cr–TiO2 sintered at 200 °C. This
high activity is ascribed to the presence of oxygen vacancies, which act
as electron-trapping sites.