論文がアクセプトされました(川俣先生・鈴木先生 共著)
Crystal Design of Polar One-Dimensional Hydrogen-Bonded Copper Coordination
Complexes
K. Takahashi, N. Hoshino, T. Takeda, K. Satomi, Y. Suzuki, S. Noro, T. Nakamura, J. Kawamata, T. Akutagawa
Dalton Trans., 2016, 45, 7153-7158
DOI : 10.1039/c5dt04865f
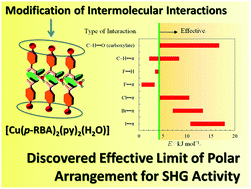
Abstract: Polar crystals exhibiting second-order harmonic generation (SHG) were
designed by adjusting the intermolecular interactions of mononuclear Cu(II)
complexes in which one H2O, two pyridines (py), and two p-substituted benzoate
(p-RBA) ligands (R = F, Cl, Br, I, CH3, and OCH3) were coordinated to a
Cu(II) ion, forming a penta-coordinated asymmetric [Cu(II)(p-RBA)2(py)2(H2O)]
mononuclear structure with a permanent dipole moment along the direction
of the Cu–OH2 coordination axis. Each asymmetric [Cu(II)(p-RBA)2(py)2(H2O)]
complex formed a polar one-dimensional hydrogen-bonded chain, [Cu(II)(p-RBA)2(py)2(H2O)]∞,
between the non-coordinated carboxylate oxygen atom of the p-RBA ligand
and the hydrogen atom of the H2O molecule. The formation of a polar crystal
depended on the arrangement of polar hydrogen-bonded chains; the parallel
arrangement of each polar chain resulted in a polar crystal. The chemical
design of the R group in the p-RBA ligand enabled tuning of the magnitude
of the interchain interactions and crystal polarity; polar crystals were
obtained using p-RBA ligands with R = Cl, Br, I, and OCH3. In contrast,
apolar crystals were grown from complexes containing p-RBA ligands with
R = F and CH3. In all crystals, a polar two-dimensional (2D) layer constructed
from the parallel polar [Cu(II)(p-RBA)2(py)2(H2O)]∞ chain arrangement was
formed based on weak van der Waals C–H⋯−O– interactions between the hydrogen
atom of py and the carboxylate oxygen atom of the p-RBA ligand. Weak interlayer
halogen (X)⋯π and multipoint C–H⋯π interactions played important roles
in forming parallel arrangements of polar 2D layers and polar crystals,
but there were no effective intermolecular interactions between the polar
2D layers in apolar [Cu(II)(p-FBA)2(py)2(H2O)] and [Cu(II)(p-CH3BA)2(py)2(H2O)]
crystals. The magnitudes of the interlayer interactions in the polar crystals
were larger than those in the apolar ones because of the effective intermolecular
interactions. The SHG intensities of the four polar crystals were approximately
0.7 times larger than that of sucrose.