論文がアクセプトされました(山崎先生)
Developing Active TiO2 Nanorods by Examining the Influence of Morphological
Changes from Nanorods to Nanoparticles on Photocatalytic Activity
Y. Yamazaki, K. Azami, R. Katoh, S. Yamazaki
ACS Appl. Nano Mater., 2018, 1, 5927-5935.
DOI: 10.1021/acsanm.8b01617
Abstract: We synthesized rutile TiO2 nanorods with high crystallinity, i.e., degree
of crystallinity greater than ca. 90%, by using a hydrothermal method and
studied the effect of the morphological change from rod shape to subsphere
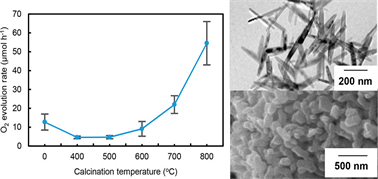
by the subsequent thermal treatment. The photocatalytic activity was estimated
for O2 evolution from water oxidation because this reaction was hardly
affected by the specific surface area of photocatalysts. Thermal desorption
and gas chromatography-mass spectrometry measurements suggested the decomposition
of residual glycolic acid in TiO2 nanorods at 272 and 590 °C. Observation
with scanning electron microscopy and physical properties such as specific
surface area and crystallite size indicated deformation of the rod shape
to subsphere by the calcination above 600 °C. Time-resolved microwave conductivity
measurements have revealed that the TiO2 nanorods possess the longest lifetime
of photogenerated electrons among all samples. However, the O2 evolution
rate of the TiO2 nanorods was much lower than subsphere TiO2 obtained by
calcination at 800 °C (TiO2-800). Photodeposition of Pt and PbO2 showed
that the oxidation and the reduction sites were separated on TiO2-800 whereas
their deposition distributed uniformly on the TiO2 nanorods. The Pt loading
on the surface of the TiO2 nanorods increased the O2 evolution rate whereas
that on TiO2-800 was hardly affected, indicating that the Pt loading enhances
the charge separation on the surface of the TiO2 nanorods. Therefore, the
lower photocatalytic activity of the TiO2 nanorods is ascribed to the recombination
process on the surface. The suppression of the recombination on the surface
and thereby the utilization of the long lifetime of the photogenerated
electrons is the key factor to develop superior TiO2 nanorods for photocatalysis.