論文がアクセプトされました(K_藤井先生)
Solvation Structure of Poly(benzyl methacrylate) in a Solvate Ionic Liquid:
Preferential Solvation of Li-Glyme Complex Cation
K. Hashimoto, Y. Kobayashi, H. Kokubo, T. Ueki, K. Ohara, K. Fujii, M. Watanabe
J. Phys. Chem. B, 2019, 123, 4098-4107.
DOI : 10.1021/acs.jpcb.9b02458
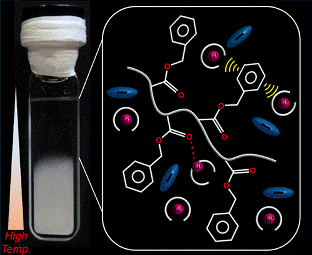
Abstract: We report the solvation structure of a lower critical solution temperature
(LCST)-type thermoresponsive polymer in a solvate ionic liquid (SIL, i.e.,
an ionic liquid comprising solvate ions) to elucidate the predominant interaction
for the dissolution of the thermoresponsive polymer in SIL at low temperatures.
The solvation structure of poly(benzyl methacrylate) (PBnMA) and a model
compound of its monomer in a typical glyme-based SIL, [Li(G4)][TFSA] (G4:
tetraglyme; TFSA: bis(trifluoromethanesulfonyl)amide), have been investigated
using high-energy X-ray total scattering and all-atom molecular dynamics
simulations. In the model compound/SIL system, the intermolecular components
extracted from the total G(r)s revealed that the ester moiety of BnMA is
preferentially solvated by Li cations through a cation–dipole interaction,
which induces slight desolvation of the G4 molecules, and the aromatic
ring of BnMA is secondarily solvated by the [Li(G4)] cation complex through
a cation−π interaction with maintaining the complex structure. In contrast,
TFSA anions are attracted only by the [Li(G4)] cation. These interactions
result in the formation of a solvation layer of SILs around the aromatic
ring, which plays a key role in the negative entropy and enthalpy of mixing.
Meanwhile, in the polymer solution, the coordination number of the Li cation
around the ester moiety significantly decreased. This could be ascribed
to the steric effect of the bulky side chains, preventing the approach
of the [Li(G4)] cation complex to the ester moiety located near the main
chain. These solvation structures lead to small absolute values of negative
entropy and enthalpy of mixing, which together are key factors to understand
the LCST-type phase behavior in the IL system.