論文がアクセプトされました(K_藤井先生)
Anion Coordination Characteristics of Ion-Pair Complexes in Highly Concentrated
Aqueous Lithium Bis(trifluoromethanesulfonyl)amide Electrolytes
T. Tsurumura, Y. Hashimoto, M. Morita, Y. Umebayashi, K. Fujii
Anal. Sci., 2019, 35, 289-294.
DOI : 10.2116/analsci.18P407
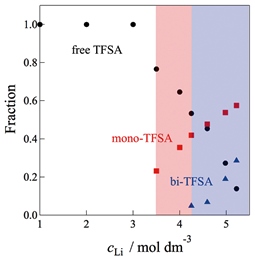
Abstract: We report on the structures of Li-ion complexes in salt-concentrated aqueous
electrolytes based on lithium bis(trifluoromethanesulfonyl)amide (LiTFSA),
particularly focusing on the anion coordination behavior of the ion-pair
complexes in the high concentration region cLi > 3.0 mol dm−3. Quantitative
data analysis of the Raman spectra revealed the following. (1) Li ions
do not coordinate with TFSA anions at lower cLi (<3.0 mol dm−3) to exist
as ion pair-free ions. (2) In the concentrated region (cLi = 3.0 – 4.0
mol dm−3), the TFSA anions coordinate as monodentate ligands (mono-TFSA)
with Li ions to form ion-pair complexes and coexist with free TFSA in the
bulk. (3) Further increasing the cLi (4.0 – 5.2 mol dm−3) results in both
monodentate and bidentate coordination (bi-TFSA) modes of TFSA anions to
Li ions, yielding complicated ion-pair complexes in the first coordination
sphere. The Walden plots, based on ionic conductivity and viscosity data,
implied that the ion-conducting mechanism in the highly salt-concentrated
region was considerably different from that in the dilute region (i.e.,
vehicle mechanism).